Instructions for use
Intended Use
ColoSense® is intended for the qualitative detection of colorectal neoplasia-associated RNA markers and for the presence of occult hemoglobin in human stool. ColoSense is for use with the ColoSense Collection Kit, the ColoSense Test Kit, the ColoSense Software, and the following instruments: Polymedco iFOBT Analyzer; bioMérieux EMAG Nucleic Acid Extraction System; and Bio-Rad QXDx ddPCR System. ColoSense is a single-site test performed at Geneoscopy, Inc.
A positive ColoSense result may indicate the presence of colorectal cancer (CRC), advanced adenoma (AA), or serrated precancerous lesions (SPL), and should be followed by a colonoscopy. ColoSense is indicated as a screening test for adults, 45 years of age or older, who are at typical average risk for developing CRC. ColoSense is not a replacement for diagnostic colonoscopy or surveillance colonoscopy in high-risk individuals.
To report any adverse events from using this kit, or if you need assistance, please call ColoSense patient services at (314) 887-7778.
Glossary
Colon: Part of the human digestive system also known as the large intestine.
Colorectal Cancer (CRC): Abnormal growth on the wall of the colon or rectum that is malignant.
Colonoscopy: A medical procedure in which a flexible instrument is inserted through the anus in order to examine the colon.
Hemoglobin: A compound in blood. Blood or hemoglobin in the stool that cannot be seen by the naked eye is referred to as occult blood.
Neoplasia: The abnormal growth. Neoplasia can be a precancerous polyp (benign) or cancerous (malignant).
Precancerous polyps: Abnormal growth on the wall of the colon or rectum that is not cancer but may develop into cancer.
RNA: Ribonucleic acid – RNA is a copy of genetic material in cells. Cells are sloughed off into the stool, releasing RNA into the stool. Analysis of the RNA in stool can provide information about whether cancerous and precancerous cells are present in the colorectum.
Summary of the Test
Screening for colorectal cancer can often find colorectal cancer early, when it’s small, hasn’t spread, and might be easier to treat. Regular screening can even prevent colorectal cancer by detecting and removing polyps before they have the chance to turn into cancer. A polyp can take as many as 10 to 15 years to develop into cancer.
ColoSense is a screening test that is designed to use a bowel movement (stool sample) to detect the presence of abnormal RNA and fecal occult blood which may be indicative of colorectal cancer and precancerous polyps.
The ColoSense Collection Kit is designed to collect your stool sample and preserve it during transport back to the laboratory for testing. After returning your stool sample to the laboratory, the laboratory will complete testing and report the results to your healthcare provider. Your provider will contact you with the test results.
NOTE: No changes to diet or medications are required for this screening test.
Principle of the Procedure
The ColoSense Test combines information about you (Attestation Form) with tests for RNA (stool sample) and blood (fecal swab) to generate a ColoSense Score. The score can tell us if you have colorectal neoplasia, which includes colorectal cancer and precancerous polyps.
Storing your Collection Kit
Store the ColoSense Collection Kit at room temperature, out of the reach of children, and out of direct sunlight until ready for use.
Contraindications
ColoSense is NOT indicated for use for patients who have the following:
- 12 months for fecal occult blood test (FOBT) or fecal immunochemical test (FIT)
- 36 months for FIT-DNA Test
- Inflammatory Bowel Disease (IBD) including chronic ulcerative colitis (CUC) and Crohn’s Disease.
- Familial adenomatous polyposis (FAP)
- Family history of colorectal cancer
- Hereditary non-polyposis colorectal cancer syndrome (HNPCC) or “Lynch Syndrome”
- Peutz-Jeghers Syndrome
- MUTYH Polyposis (MAP)
- Gardner’s Syndrome
- Turcot’s (or Crail’s) Syndrome
- Cowden’s Syndrome
- Juvenile Polyposis
- Cronkhite-Canada Syndrome,
- Neurofibromatosis
- Familial Hyperplastic Polyposis
Before You Start: Warnings and Precautions
Do NOT provide a sample for the ColoSense test if you have the following:
- Bleeding hemorrhoids
- Bleeding cuts or wounds on your hands
- Rectal bleeding
- Menstrual bleeding (period)
- Diarrhea
No colorectal cancer screening test is perfect. ColoSense may produce false negative or false positive results. Based on the clinical validation data for ColoSense, with 25 / 27 colorectal cancers from typical average risk patients identified (92.6%) and with a 95% two-sided confidence interval lower bound of 75.7%, there is a chance that as many as 24.3% of patients with colorectal cancer may be missed by this test, given the current data available.
- A false positive (incorrect) result occurs when ColoSense produces a positive result when colorectal cancer or precancers are not present.
- A false negative (incorrect) result may occur when ColoSense does not detect colorectal cancer or precancers that are present in the colon or rectum.
- A positive test result indicates that colorectal cancer or precancers may be present in the colon or rectum. It is recommended that positive results be followed up by a colonoscopy.
- A negative test result indicates colorectal cancer or precancers were not detected.
- Benefits and risks with repeated ColoSense testing over an established period of time has not been studied.
- Do NOT drink the bottle of Sample Preservative and keep away from children.
- Do NOT let the liquid touch your skin or eyes. If the liquid touches your skin or eyes, rinse thoroughly with water.
- Do NOT apply green top sample probe to sample after pouring on Sample Preservative.
- Do NOT mix components from different Collection Kits.
- Fecal samples should be treated as if they are potentially infectious.
- The Instructions for Use must be followed carefully for accurate results.
- Opening or closing lids in the kit may be difficult for some. Be careful to avoid hand strain and ensure containers are properly sealed prior to shipping.
- Ensure the attestation form is complete and placed in box for return shipment.
- Samples must be received by the laboratory within 96 hours of collection to be suitable for testing. Ensure samples are shipped within 24 hours following collection.
ColoSense Collection Kit Contents

A Attestation Form | F Resealable Bag 2 with Absorbent Pad |
B Toilet Seat Bracket | G Collection Kit Instructions for Use |
C Resealable Bag 1 | H Sample Preservative The 275mL Sample Preservative contains less than 10% EDTA in a Tris buffered solution. |
D Stool Container | I OC-Auto Sampling Bottle The 2mL OC Auto Sampling Bottle contains a HEPES buffered solution. |
E Stool Container Lid | J Resealable Bag 3 |
Collecting Your Stool Sample
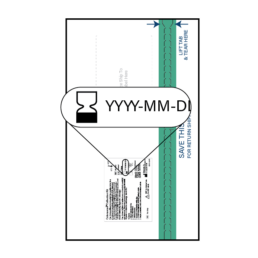
1.
Check the expiration date (YYYY-MM-DD) on the label on the outside of the Collection Kit to ensure the kit is NOT expired. If the kit is expired, please contact ColoSense patient services at (314) 887-7778.
2.
Samples should be shipped back within 24 hours of collection. Perform the following steps only if you can ship your sample within the next 24 hours.
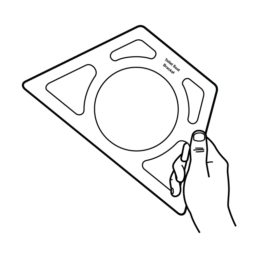
3.
Remove Toilet Seat Bracket from box.
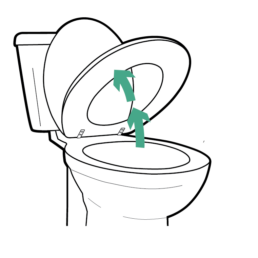
4.
Raise toilet seat and lid.
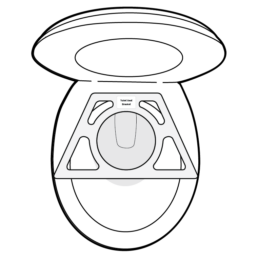
5.
Place Toilet Seat Bracket onto the toilet bowl so that it sits on top of the bowl and beneath the seat with the short end towards the back.

6.
Lower toilet seat down over Toilet Seat Bracket making sure the circular opening of bracket is visible.
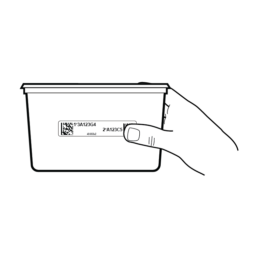
7.
Remove Stool Container from box.
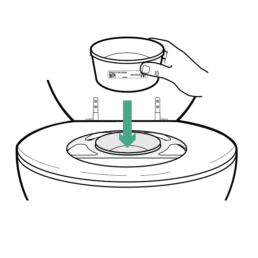
8.
Place Stool Container into bracket opening.
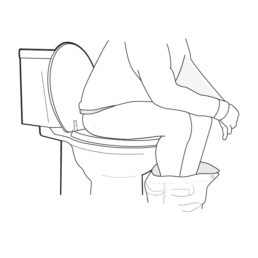
9.
Sit on the toilet and have a bowel movement into the container.
Do NOT collect urine. Do NOT collect bloody, loose, orpelleted stool.
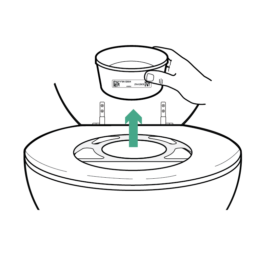
10.
Once finished lift Stool Container from bracket and place on a level, stable surface. The Toilet Seat Bracket can be recycled, disposed of, or shipped back in your collection kit.
Do NOT seal Stool Container before swabbing sample and applying Sample Preservative.
11.
Remove OC-Auto Sampling Bottle from box.
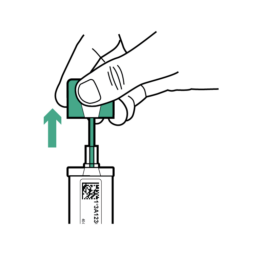
12.
While holding the bottle in an upright position (contains liquid Sample Preservative), pull upwards to open.
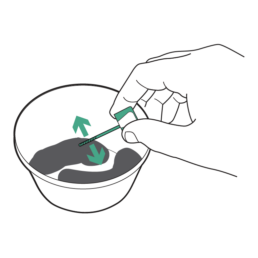
13.
Randomly scrape several sections of the stool sample with the sample probe. Ensure the grooved portion is completely covered in stool.
Do NOT scrape stool after addition of Sample Preservative. If Sample Preservative is added prior to using the sample probe, contact ColoSense patient services for a new Collection Kit.
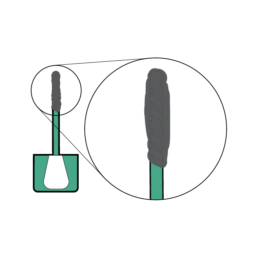
14.
Confirm the grooved portion of the sample probe is completely covered before closing.
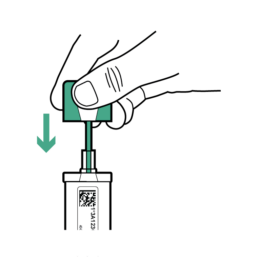
15.
Close OC-Auto Sampling Bottle by re-inserting the sample probe into the bottle and pressing down until the cap seals on. Shake the bottle gently a few times to mix the stool sample with the Sample Preservative. The bottle is finished and can be set aside for now.
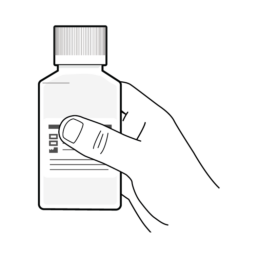
16.
Remove the bottle of Sample Preservative from the box.
NOT for internal use. Do NOT drink.
Do NOT add Sample Preservative before using the OC-Auto Sampling Bottle to scrape the sample.
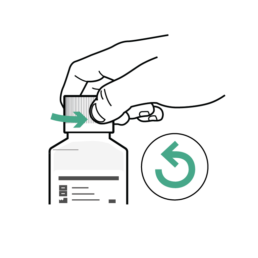
17.
Turn the bottle cap counterclockwise to open.
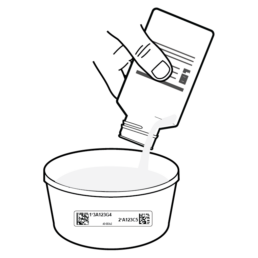
18.
Pour Sample Preservative into Stool Container to completely cover the stool sample.
Pour the Sample Preservative into the Stool Container. Use the entire bottle or enough to cover the stool sample.

19.
Return the cap to the top of the Sample Preservative and turn the cap clockwise to close. Set bottle back in the box.
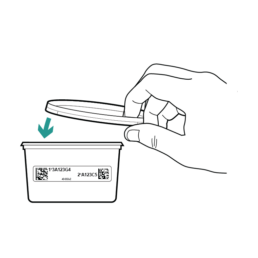
20.
Place the Stool Container Lid on top of the sample container.
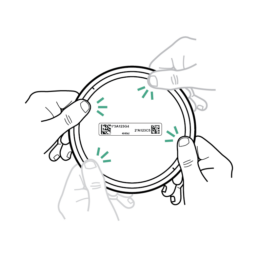
21.
Press down tightly on the Stool Container Lid until feeling the container seal. Press down around the edges until you feel the lid seal onto the bucket.
Do NOT pull tabs on side of lid or attempt to reopen container.
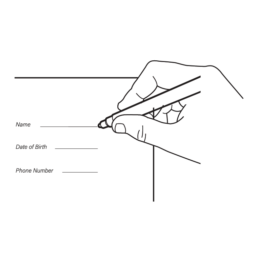
22.
Fill in all required information on the Attestation Form.
NOTE: A complete Attestation Form is required to process your sample.

23.
Insert OC-Auto Sampling Bottle into Bag 3. Remove air as best you can and seal bag.
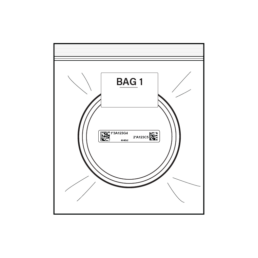
24.
Insert Stool Container into Resealable Bag 1. Remove all air from bag as best as you can and seal bag.
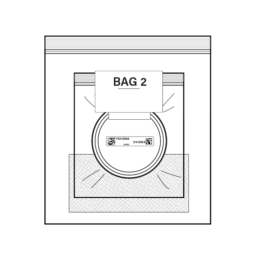
25.
Insert Bag 1 with the closed Stool Container into Bag 2. Remove all air from bag as best as you can and seal bag.
NOTE: Please do not remove Absorbent Pad from Bag 2.

26.
Place each of the following into the shipping box. Any remaining materials can be discarded.
- Stool Container in Sealed Bags
- Sample Preservative
- OC-Auto Sample Preservative Bottle in Bag 3
- Completed Attestation Form
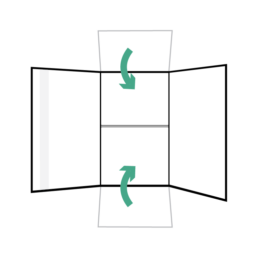
27.
Fold smaller flaps of box over first.
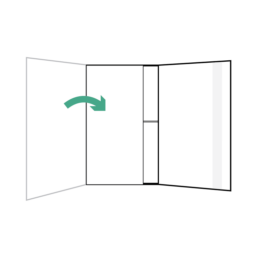
28.
Fold flap without adhesive strip over the two smaller flaps.
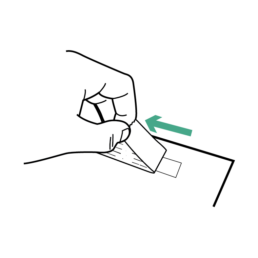
29.
Remove adhesive strip from box to expose adhesive.
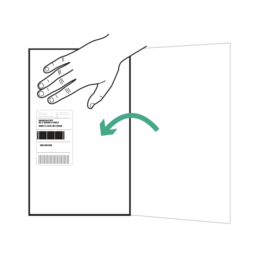
30.
Fold flap with adhesive strip and make sure the FedEx return label is visible on top. Press down to seal.
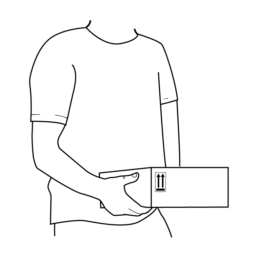
31.
Deliver to nearest FedEx location within 24 hours.
Visit Fedex.com or call:
1 (800) 463-3339 to see drop-off locations near you.
Shipping Your Stool Sample
| Date collected | Last date to be shipped (before noon) |
|---|---|
| Sunday | Monday |
| Monday | Tuesday |
| Tuesday | Wednesday |
| Wednesday | Thursday |
| Thursday | Friday |
| Friday* | Friday |
| Saturday* | Saturday |
Samples must be shipped so they arrive at the laboratory within 4 days of collection to be tested. Ship your samples the same day of collection to ensure your sample can be tested.
*FedEx may not pickup or deliver on Saturday or Sunday
Understand Your ColoSense Results
Contact your healthcare provider to discuss your results. The test result can be Positive, Negative, or No result obtained.
Positive Result
- A POSITIVE result means the test detected abnormal RNA and/or blood that could be caused by cancer or precancer in the colon or rectum.
- A positive test result is recommended to be followed up by a colonoscopy.
- A false positive (incorrect) result is possible and means the test result is positive even though no cancer or precancer is present.
Negative Result
- A NEGATIVE result means the test did not detect abnormal RNA or blood in the sample.
- A negative test result does not guarantee the absence of cancer or precancer. You should continue with a regular screening schedule.
- A false negative (incorrect) result is possible and means the test result missed a potential cancer or precancer.
- Talk about your test result with your doctor.
No Result
- A no result or invalid result means the test was not able to produce a result.
- When this occurs, consult your doctor about next steps, which may include potentially having to provide another sample.
ColoSense Collection Kit Symbols Used
| REF Symbol | Manufacturer Information | ||
| Lot Number | Caution | ||
| Expiration Date | Temperature Storage Range | ||
| Read Instructions | Number of Tests in the Kit | ||
| In Vitro Diagnostic Medical Device | Do Not Reuse | ||
| Medical Prescription | Serial Number |